Quiz Summary
0 of 20 questions completed
Questions:
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- 11
- 12
- 13
- 14
- 15
- 16
- 17
- 18
- 19
- 20
Information
|
You must specify a text. |
|
|
You must specify a text. |
You have already completed the quiz before. Hence you can not start it again.
Quiz is loading…
You must sign in or sign up to start the quiz.
You must first complete the following:
Results
Results
0 of 20 questions answered correctly
Your time:
Time has elapsed
You have reached 0 of 0 point(s), (0)
Earned Point(s): 0 of 0, (0)
0 Essay(s) Pending (Possible Point(s): 0)
Categories
- Not categorized 0%
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- 11
- 12
- 13
- 14
- 15
- 16
- 17
- 18
- 19
- 20
- Answered
- Review
-
Question 1 of 20
1. Question
1 Diagram 1 shows the paper chromatogram of substance X.
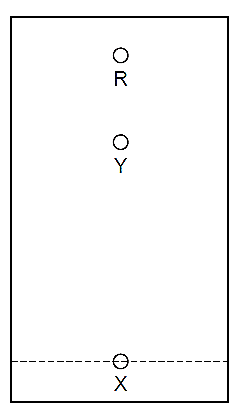
Diagram 1
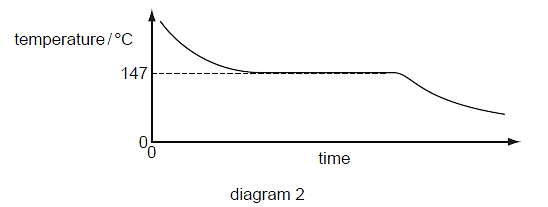
Diagram 2 shows the cooling curve for substance Y.
Which statement about X and Y is correct?
A X and Y are both mixtures.
B X and Y are both pure substances.
C X is a mixture and Y is a pure substance.
D X is a pure substance and Y is a mixture.
CorrectIncorrect -
Question 2 of 20
2. Question
2 A student wishes to extract the colouring from some orchid flowers to make an indicator solution.
Which of these instructions should the student follow?
- crush the orchid flower
- add acid
- add a solvent
- filter the mixture
A 1, 2 and 3
B 1, 2 and 4
C 1, 3 and 4
D 2, 3 and 4
CorrectIncorrect -
Question 3 of 20
3. Question
3 The graph below shows the change in temperature with time when steam at 120 °C is cooled to –20 °C.
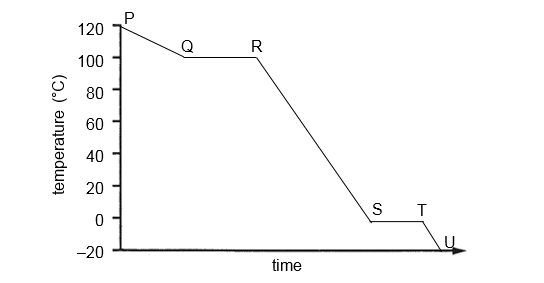
Which of the following shows the correct change taking place between the points?
points
change
A
P to Q
average energy of particles is increasing
B
Q to R
steam is condensing
C
R to S
volume of water is increasing
D
T to U
ice is forming
CorrectIncorrect -
Question 4 of 20
4. Question
4 Which statement best describes a mixture?
A A mixture can only be separated into its components by chemical methods.
B A mixture can only exist in one particular state of matter.
C A mixture’s chemical properties are the same as those of its components.
D A mixture is only made up of elements combined in a fixed proportion.
CorrectIncorrect -
Question 5 of 20
5. Question
5 The formulae of three substances are shown.
substance formula carbon dioxide CO2 ethene C2H4 nitrogen N2 Which statement is correct?
A Carbon dioxide is made up of two different types of atom hence it is a mixture.
B Ethene and carbon dioxide are mixtures because their elements are not combined in a fixed ratio of 1:1.
C Nitrogen is made up of two atoms hence it is a compound.
D Nitrogen is made up of only one type of atom hence it is an element.
CorrectIncorrect -
Question 6 of 20
6. Question
6 How many electrons and protons are present in an ion of nitrogen?
number of electrons
number of protons
A
7
7
B
10
7
C
7
14
D
10
14
CorrectIncorrect -
Question 7 of 20
7. Question
7 The table below shows the properties of some substances.
Which substance has intermolecular forces of attraction between its particles?
melting point / °C
electrical conductivity
in solid state
in liquid state
A
-42
does not conduct
does not conduct
B
960
conduct
conduct
C
1326
does not conduct
conduct
D
2220
conduct
does not conduct
CorrectIncorrect -
Question 8 of 20
8. Question
8 Rain water contains dissolved carbon dioxide.
What is the pH value of rainwater?
A 5B 7
C 9
D 11
CorrectIncorrect -
Question 9 of 20
9. Question
9 The oxide of an element Z was added separately to sulfuric acid and aqueous ammonia.
The word equations for the reactions are shown.
Z oxide + sulfuric acid → no reaction
Z oxide + aqueous ammonia → no reaction
Which row describes Z and its oxide?
Z
Z oxide
A
metal
basic
B
metal
neutral
C
non-metal
acidic
D
non-metal
neutral
CorrectIncorrect -
Question 10 of 20
10. Question
10 The table below shows some properties of four metals.
Which metal is in Group I of the Periodic Table?
density
hardness
A
high
hard
B
high
soft
C
low
hard
D
low
soft
CorrectIncorrect -
Question 11 of 20
11. Question
11 Which of the following is observed when liquid bromine is added to aqueous potassium chloride?
A A brown vapour is formed.
B A silvery solid is formed.
C A greenish-yellow gas is produced.
D There is no visible reaction.
CorrectIncorrect -
Question 12 of 20
12. Question
12 The diagrams below show the results obtained when three different metallic discs of the same shape and size were dropped into dilute hydrochloric acid separately.
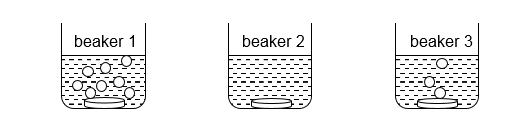
Which metal is likely to have been placed in each beaker?
beaker 1
beaker 2
beaker 3
A
magnesium
copper
calcium
B
calcium
copper
magnesium
C
copper
magnesium
calcium
D
calcium
magnesium
copper
CorrectIncorrect -
Question 13 of 20
13. Question
13 Nickel is between iron and copper in the reactivity series.
Which of the following statement(s) can be deduced from its position in the reactivity series?
I Nickel forms effervescence with cold water.
II Nickel is obtained by heating nickel ore with carbon monoxide.
III Nickel reacts with dilute hydrochloric acid to produce hydrogen gas.
A I only
B I and III
C II and III
D I, II and III
CorrectIncorrect -
Question 14 of 20
14. Question
14 Which of the following is not a reason for recycling metals?
A Metal ores are a finite resource.
B Recycling increases the purities of metals.
C Recycling reduces the cost of producing metals.
D Recycling reduces the air pollution.
CorrectIncorrect -
Question 15 of 20
15. Question
15 Four shiny steel paper-clips are placed in test-tubes and left for seven days.
In which test-tube does the paper-clip rust?
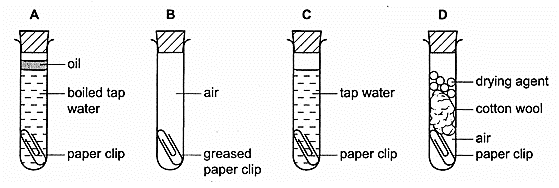 CorrectIncorrect
CorrectIncorrect -
Question 16 of 20
16. Question
16 Which pie chart shows the proportions of gases in clean air?
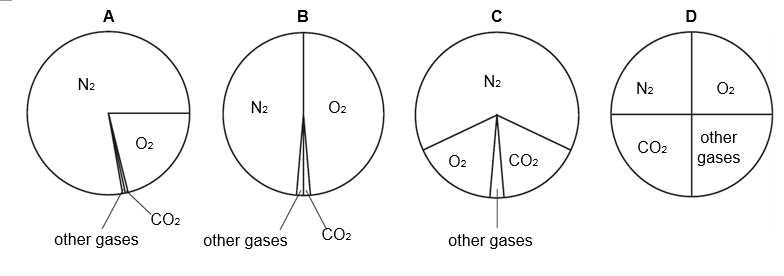 CorrectIncorrect
CorrectIncorrect -
Question 17 of 20
17. Question
17 Carbon monoxide, sulfur dioxide and oxides of nitrogen are all common pollutants of air.
Which pollutant is shown with its correct source and adverse effect on the environment?
pollutant
source
effect on the environment
A
carbon monoxide
combustion of fossil fuels
acid rain
B
carbon monoxide
lightning
global warming
C
oxides of nitrogen
lightning
acid rain
D
sulfur dioxide
volcanoes
global warming
CorrectIncorrect -
Question 18 of 20
18. Question
18 The diagram shows an industrial process used to produce substance M.
Substance M is used as a fuel for heavy vehicles and ships.
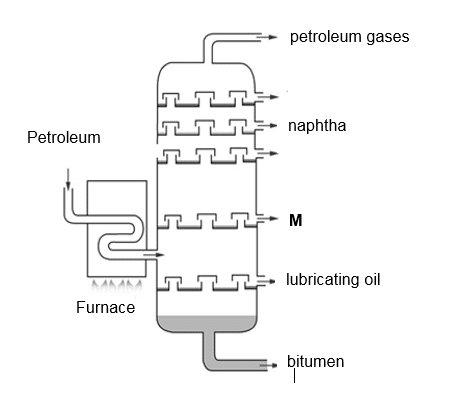
What is this process and what is substance M?
process
substance M
A
fractional distillation
gasoline
B
fractional distillation
diesel
C
thermal decomposition
petrol
D
thermal decomposition
diesel
CorrectIncorrect -
Question 19 of 20
19. Question
19 What is the structure of the product formed when ethene gas is passed through aqueous bromine?
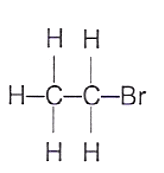
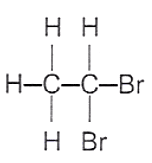
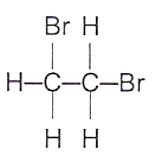
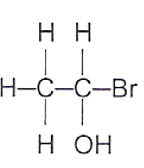
A B C D
CorrectIncorrect -
Question 20 of 20
20. Question
20 A chemist carried out a cracking reaction on a hydrocarbon, X, and obtained two products, Y and Z.
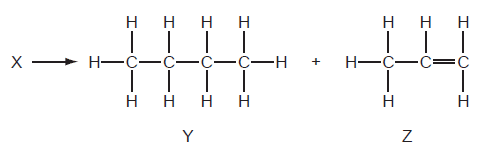
The chemist then wrote the following statements in his notebook.
- A molecule of X has 7 carbon atoms.
- Y is unsaturated.
- Z will decolourised aqueous bromine easily.
- The conditions for cracking are Al2O3 catalyst, 200 °
Which statements are correct?
A 3 and 4
B 1, 2 and 4
C 1 and 3
D 1, 2 and 3
CorrectIncorrect
