Quiz Summary
0 of 21 questions completed
Questions:
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- 11
- 12
- 13
- 14
- 15
- 16
- 17
- 18
- 19
- 20
- 21
Information
|
You must specify a text. |
|
|
You must specify a text. |
You have already completed the quiz before. Hence you can not start it again.
Quiz is loading…
You must sign in or sign up to start the quiz.
You must first complete the following:
Results
Results
0 of 21 questions answered correctly
Your time:
Time has elapsed
You have reached 0 of 0 point(s), (0)
Earned Point(s): 0 of 0, (0)
0 Essay(s) Pending (Possible Point(s): 0)
Categories
- Not categorized 0%
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- 11
- 12
- 13
- 14
- 15
- 16
- 17
- 18
- 19
- 20
- 21
- Answered
- Review
-
Question 1 of 21
1. Question
A thermometer is placed in water and the temperature measured is 43OC.
When an unknown solid X is added into the water, an endothermic change takes place and the temperature is observed to change by 4.5OC.
What is the final temperature measured?
A 38.0°C B 38.5°C
C 47.0°C D 47.5°C
CorrectIncorrect -
Question 2 of 21
2. Question
Which one of the following has the same volume as one mole of fluorine gas?
CorrectIncorrect -
Question 3 of 21
3. Question
A student performs a series of experiments using catalysts. For each experiment he uses the same amount of reactants and the same amount of catalyst. He plots the graphs below from the results.
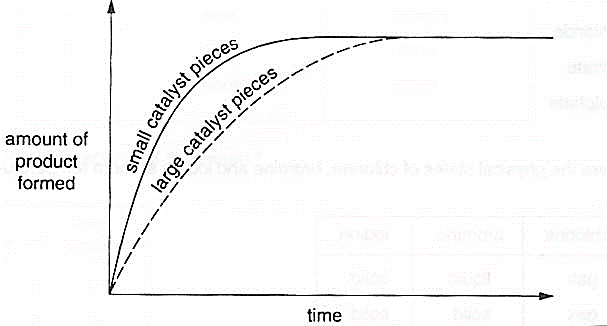
Which of the following best describe the graphs?
CorrectIncorrect -
Question 4 of 21
4. Question
4. Part of some chemical reactions are shown.
Which reaction represents oxidation?A Mg(s) → Mg2+(aq) B Cl2(g) → 2Cl–(aq)
C CO2(g) → C(s) D Fe3+(aq) → Fe2+(aq)
CorrectIncorrect -
Question 5 of 21
5. Question
48 dm3 of hydrogen is ignited in air.
2H2 + O2 → 2H2O
What is the mass of water formed?
CorrectIncorrect -
Question 6 of 21
6. Question
The reaction between iron(III) ions and iodide ions is represented by the equation.
2Fe3+ (aq) + 2I– (aq) → 2Fe2+ (aq) + I2 (aq)
Which statement is correct?
A
Fe3+ ions are oxidised by loss of electrons.
B
Fe3+ ions are reduced by gain of electrons.
C
I– ions are oxidised by gain of electrons.
D
I– ions are reduced by loss of electrons.
CorrectIncorrect -
Question 7 of 21
7. Question
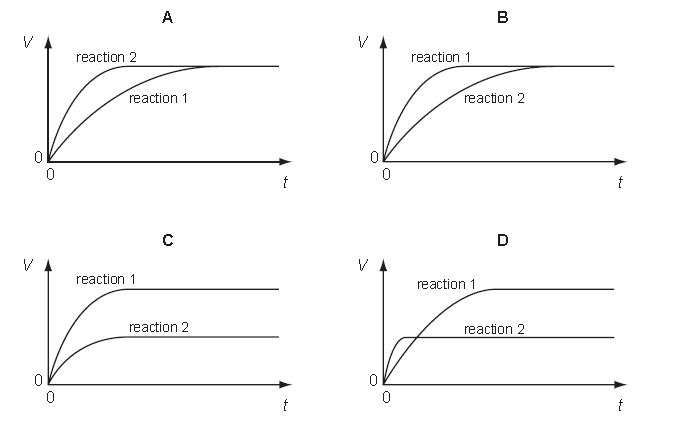
A student performs two reactions.
reaction 1: 10 g of magnesium ribbon with excess 2.0 mol/dm3 dilute hydrochloric acid.
reaction 2: 5 g of magnesium powder with excess 2.0 mol/dm3 dilute hydrochloric acid.
In both experiments, the volume of hydrogen produced, V, is measured against time, t, and the results plotted graphically.
Which set of graphs is correct?
 CorrectIncorrect
CorrectIncorrect -
Question 8 of 21
8. Question
Propane burns completely in oxygen as shown in the equation.
C3H8 (g) + 5O2 (g) → 3CO2 (g) + 4H2O (l)
If 0.3 mol of propane is burnt completely, what is the volume of gaseous product obtained at room temperature and pressure?
A
0.3 dm3
B
0.9 dm3
C
7.2 dm3
D
21.6 dm3
CorrectIncorrect -
Question 9 of 21
9. Question
The following graph was obtained when potassium fluoride was dissolved in water.
Which of the following statements about the reaction is correct?
CorrectIncorrect -
Question 10 of 21
10. Question
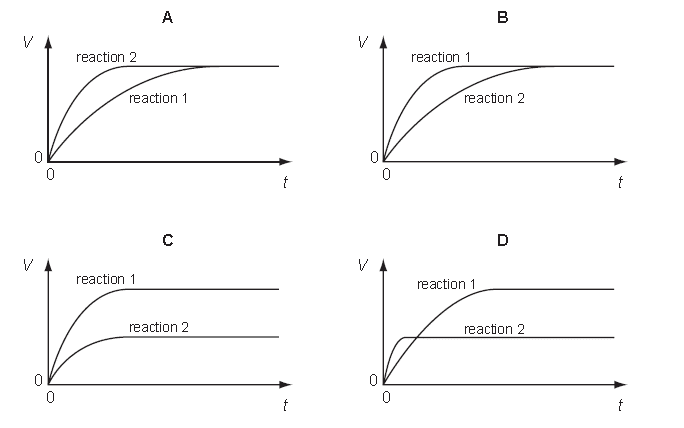
10 A student performs two reactions.
Reaction 1: 5 g of magnesium ribbon with excess 2.0 mol/dm3 dilute hydrochloric acid
Reaction 2: 5 g of magnesium powder with excess 2.0 mol/dm3 dilute hydrochloric acid
In both experiments, the volume of hydrogen produced, V, is measured against time, t, and the results plotted graphically.
 CorrectIncorrect
CorrectIncorrect -
Question 11 of 21
11. Question
In which pair of compounds is the underlined element in the same oxidation state?
A
CuCl2 and NaCl
B
FeO and Fe2(SO4)3
C
H2S and SO2
D
MnO2 and MnCl2
CorrectIncorrect -
Question 12 of 21
12. Question
The reaction between calcium and aqueous zinc nitrate gives out heat energy.
Which energy level diagram accurately represents the reaction?
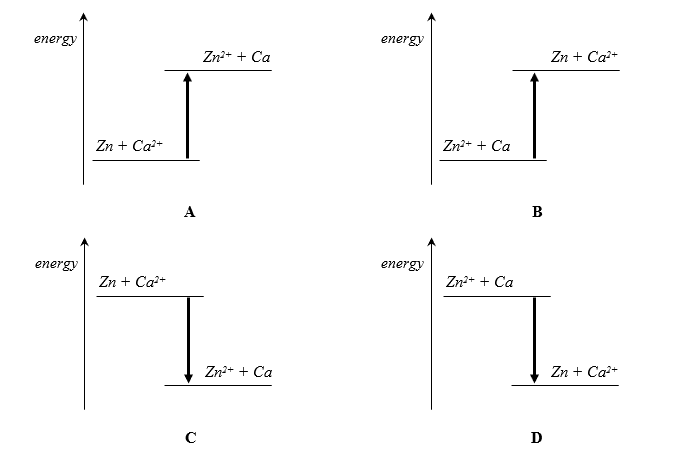 CorrectIncorrect
CorrectIncorrect -
Question 13 of 21
13. Question
In which substance does carbon have the smallest oxidation number?
A
C
B
CO
C
CO2
D
CaCO3
CorrectIncorrect -
Question 14 of 21
14. Question
In the graph shown below, curve Y represents the results of reacting excess of magnesium powder with 25 cm3 of 1.0 mol / dm3 sulfuric acid at 40 °C.
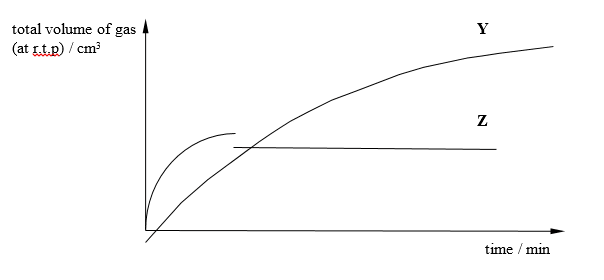
Which changes could produce curve Z?
A
Using 12.5 cm3 of 1.0 mol / dm3 sulfuric acid at 20 °C.
B
Using 12.5 cm3 of 1.0 mol / dm3 sulfuric acid at 60 °C.
C
Using 25 cm3 of 1.0 mol / dm3 sulfuric acid at 20 °C.
D
Using 25 cm3 of 1.0 mol / dm3 sulfuric acid at 60 °C.
CorrectIncorrect -
Question 15 of 21
15. Question
30 cm3 of 1.0 mol/dm3 of aqueous sodium hydroxide neutralises 25 cm3 of dilute hydrochloric acid.
NaOH + HCl → NaCl + H2O
What is the concentration of dilute hydrochloric acid?
A
0.80 mol/dm3
B
1.00 mol/dm3
C
1.20 mol/dm3
D
1.25 mol/dm3
CorrectIncorrect -
Question 16 of 21
16. Question
Which graph represents the change in mass of flask against time when a conical flask containing aqueous sodium carbonate reacts with dilute nitric acid?
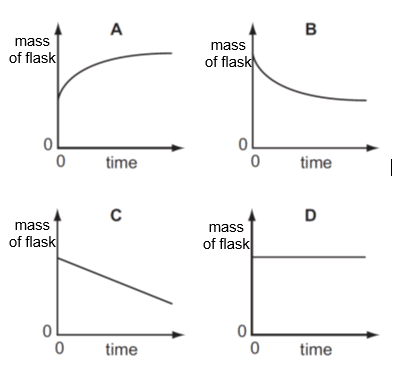 CorrectIncorrect
CorrectIncorrect -
Question 17 of 21
17. Question
2 g of calcium carbonate granules reacts with excess 2.0 mol/dm3 of dilute hydrochloric acid.
Which condition decreases the rate of the reaction?
A
Increasing the volume of dilute hydrochloric acid.
B
Using 1.0 mol/dm3 of dilute hydrochloric acid.
C
Using 5 g mass of calcium carbonate.
D
Using powdered calcium carbonate.
CorrectIncorrect -
Question 18 of 21
18. Question
Which reaction is not a redox reaction?
CorrectIncorrect -
Question 19 of 21
19. Question
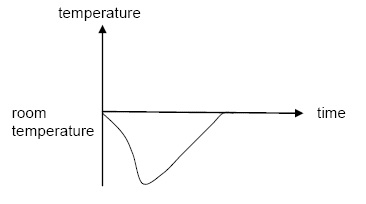
Dissolving ammonium nitrate in water is endothermic.
Which graph shows how the temperature alters as the ammonium nitrate is added to water and then the solution is left to stand?
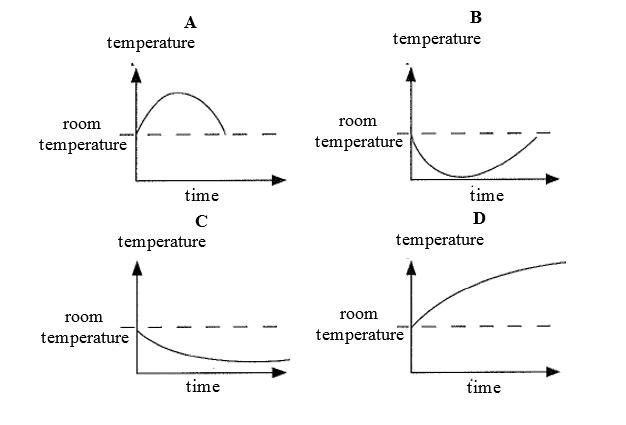 CorrectIncorrect
CorrectIncorrect -
Question 20 of 21
20. Question
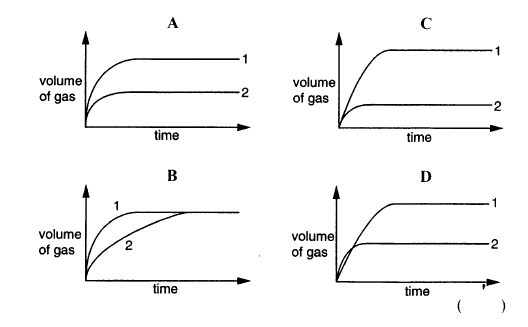
Calcium carbonate was reacted with an excess of dilute hydrochloric acid at room temperature.
CaCO3 + 2HCl → CaCl2 + H2O + CO2
Two experiments were carried out.
Experiment 1: 100g of calcium carbonate in lumps
Experiment 2 : 50g of calcium carbonate as fine powder
Which of the graphs is correct?
 CorrectIncorrect
CorrectIncorrect -
Question 21 of 21
21. Question
In which reaction is the underlined substance behaving as an oxidising agent?
A C (s) + CO2 (g) → 2CO (g)
B Cl2 (g) + 2I– (aq) → I2 (aq) + 2Cl– (aq)
C Mg (s) + CuSO4 (aq) → MgSO4 (aq) + Cu (s)
D NaOH (aq) + HNO3 (aq) → NaNO3 (aq) + H2O (l)
CorrectIncorrect
