Quiz Summary
0 of 20 questions completed
Questions:
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- 11
- 12
- 13
- 14
- 15
- 16
- 17
- 18
- 19
- 20
Information
|
You must specify a text. |
|
|
You must specify a text. |
You have already completed the quiz before. Hence you can not start it again.
Quiz is loading…
You must sign in or sign up to start the quiz.
You must first complete the following:
Results
Results
0 of 20 questions answered correctly
Your time:
Time has elapsed
You have reached 0 of 0 point(s), (0)
Earned Point(s): 0 of 0, (0)
0 Essay(s) Pending (Possible Point(s): 0)
Categories
- Not categorized 0%
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- 11
- 12
- 13
- 14
- 15
- 16
- 17
- 18
- 19
- 20
- Answered
- Review
-
Question 1 of 20
1. Question
1 Which equation shows the removal of impurities during the extraction of iron?
A C + O2 → CO2 B CaCO3 → CaO + CO2 C CaO + SiO2 → CaSiO3 D CO2 + C → 2 CO CorrectIncorrect -
Question 2 of 20
2. Question
2 Fluorine is the first element in Group VII of the Periodic Table.
Which statement about fluorine is not correct?
A
Fluorine exists as diatomic molecules.
B
Fluorine forms negative ions.
C
Fluorine is less reactive than chlorine.
D
Fluorine is pale yellow.
CorrectIncorrect -
Question 3 of 20
3. Question
3 Some copper turnings were heated with 100 cm3 of a sample of air taken from a diving bottle. After the experiment, the volume of air present was 90 cm3.
What is the percentage of oxygen present in the sample of air?
A
10%
B
20%
C
30%
D
40%
CorrectIncorrect -
Question 4 of 20
4. Question
4 Element X can form an ion X+ with an electronic structure of 2. 8. 8.
Which statements about element X are correct?1: X reacts vigorously with water to form an alkali.
2: X is an unreactive non-metal.
3: X can be cut easily with a knife.
4: X is a diatomic coloured substance.A
1 and 2
B
1 and 3
C
2 and 4
D
1, 3 and 4
CorrectIncorrect -
Question 5 of 20
5. Question
5 The graph below shows the trend of a property of the elements in Period 3.
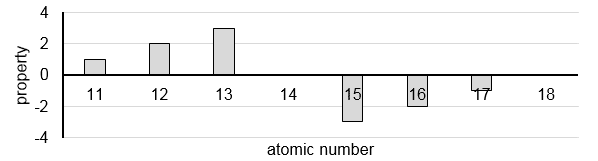
What is the property?
A
charge of ions
B
ease of gaining electrons
C
number of electron shells
D
number of valence electrons
CorrectIncorrect -
Question 6 of 20
6. Question
6 An experiment was set up as shown below to investigate the rate of rusting under different conditions.
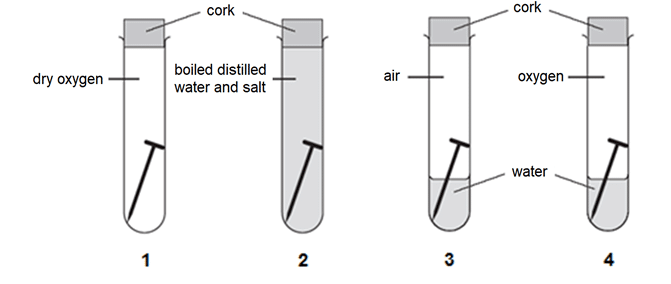
Predict the order of the test-tubes in which rust would first appear.
A
1, 2, 3, 4
B
2, 4, 3, 1
C
4, 1, 3, 2
D
4, 3, 2, 1
CorrectIncorrect -
Question 7 of 20
7. Question
7 The gases present in the exhaust gases from a motorcar engine include argon, carbon dioxide, carbon monoxide, methane, nitrogen, nitrogen dioxide and water vapour.
Which of these gases are also found in unpolluted air?A
argon, carbon dioxide, nitrogen and water vapour only
B
argon, methane, nitrogen and water vapour only
C
carbon dioxide, methane and nitrogen
D
carbon dioxide, nitrogen, nitrogen dioxide and water vapour only
CorrectIncorrect -
Question 8 of 20
8. Question
8
Which of the following diagrams shows the arrangement of the atoms in stainless steel?
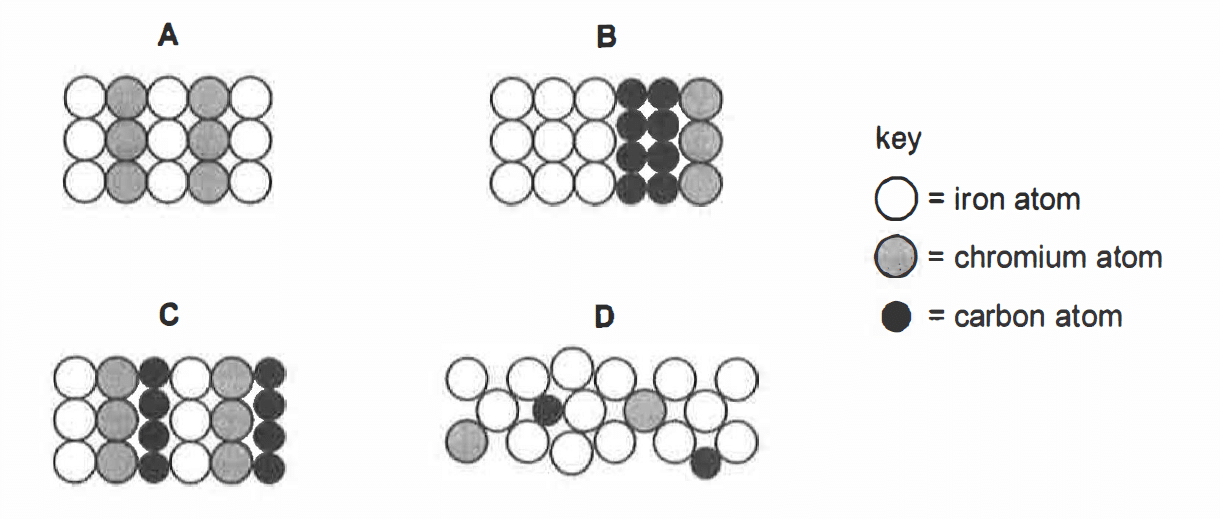 CorrectIncorrect
CorrectIncorrect -
Question 9 of 20
9. Question
9 Metals W, X, Y and Z are placed in salt solutions as shown in the table
result of placing metal in solution of
salt of W
salt of X
salt of Y
salt of Z
W
no reaction
X displaced
Y displaced
no reaction
X
no reaction
no reaction
no reaction
no reaction
Y
no reaction
X displaced
no reaction
no reaction
Z
W displaced
X displaced
Y displaced
no reaction
What is the order of reactivity of the metals from most reactive to least reactive?
A
Y → X → W → Z
B
Y → W → Z → X
C
Z → W → Y → X
D
Z → Y → X → W
CorrectIncorrect -
Question 10 of 20
10. Question
10 Which of the following shows the correct trends down the group for the melting point, density and atomic radius of alkali metals?
melting point
density
atomic radius
A
increasing
decreasing
increasing
B
decreasing
increasing
increasing
C
increasing
increasing
decreasing
D
decreasing
decreasing
decreasing
CorrectIncorrect -
Question 11 of 20
11. Question
11 The table shows the results of halogen displacement experiments.
halogen added
halide solution
X
Y
Z
X2
Y2 displaced
Z2 displaced
Y2
no reaction
no reaction
Z2
no reaction
Y2 displaced
What are halogens X, Y and Z?
X
Y
Z
A
Cl
F
Br
B
Cl
Br
F
C
F
Cl
Br
D
F
Br
Cl
CorrectIncorrect -
Question 12 of 20
12. Question
12 Many properties of an element and its compounds can be predicted from the position of the element in the Periodic Table.
What property could not be predicted in this way?A
The formula of its oxide
B
The number of isotopes it has
C
The acidic or basic nature of its oxide
D
The metallic or non-metallic properties
CorrectIncorrect -
Question 13 of 20
13. Question
13 A new halogen Z is discovered. Its relative atomic mass was found to be 370.
Which properties is Z most likely to have?A
black solid, cannot conduct electricity
B
dark green gas, soluble in water
C
grey liquid, violent reaction with water
D
white solid, no reaction with water
CorrectIncorrect -
Question 14 of 20
14. Question
14 The experiments were carried out to determine the reactivity of three metals P, Q and R.
The following table shows the results of the experiments.
Experiment
Results
P
Q
R
Reaction with HCl
Yes
No
Yes
Reduction by carbon
Yes
Yes
No
Which of the following is in the increasing order of reactivity of metals P, Q and R?
A
P, R, Q
B
Q, R, P
C
P, Q, R
D
Q, P, R
CorrectIncorrect -
Question 15 of 20
15. Question
15 Iron filings are wrapped in a damp cloth and left to rust in the apparatus as shown.
Which letter indicates the water level when rusting has completed?
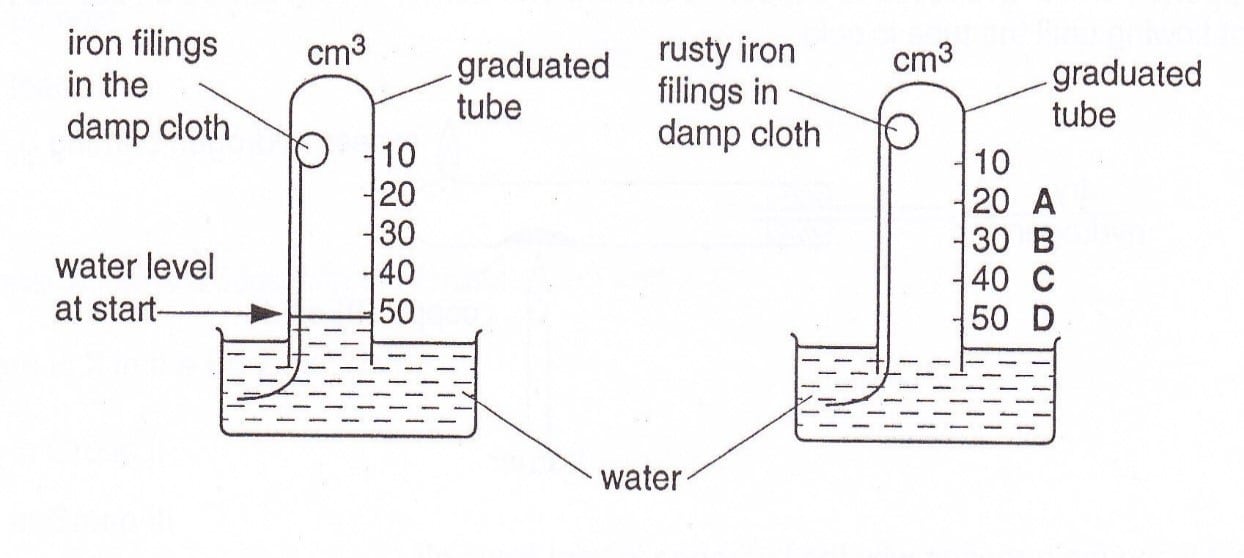 CorrectIncorrect
CorrectIncorrect -
Question 16 of 20
16. Question
16 The apparatus setup below shows the reaction between substance X and the vapour produced from boiling water, to produce gas Y.
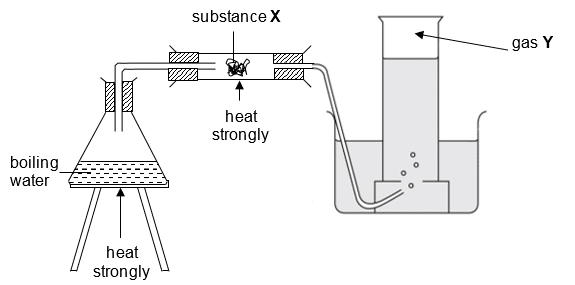
What could be X and Y?
X
Y
A
calcium
oxygen
B
copper
hydrogen
C
magnesium
hydrogen
D
zinc
water vapour
CorrectIncorrect -
Question 17 of 20
17. Question
17 Elements X and Y are in Group VII of the Periodic Table.
X is a liquid at room temperature. Y is a solid at room temperature
- Atoms of Y have more protons than atoms of X.
- Molecules of Y have more atoms than molecules of X.
- Y displaces X from aqueous solutions of X−
Which statement(s) is/are correct?
A 1 only
B 2 only
C 3 only
D 1, 2 and 3
CorrectIncorrect -
Question 18 of 20
18. Question
18 A simplified version of the Periodic Table below shows 4 elements with the code letters, W, X, Y and Z.

Which statement regarding W, X, Y and Z is correct?
A
W and X are solids but Y and Z are gases at room temperature.
B
W and X are in the same group while Y and Z are in another group.
C
W and X are in the same period while Y and Z are in another period.
D
W is a non-metal but X, Y and Z are metals.
CorrectIncorrect -
Question 19 of 20
19. Question
19 Each of the halogens X2, Y2 and Z2 was added to samples of aqueous solutions containing the ions of the other two halogens.
The table shows the results.
halogen
aqueous solution containing X–
aqueous solution containing Y–
aqueous solution containing Z–
X2
–
Y2 displaced
Z2 displaced
Y2
no reaction
–
no reaction
Z2
no reaction
Y2 displaced
–
What is the order of reactivity of the halogens?
most reactive least reactive
A
X2
Y2
Z2
B
X2
Z2
Y2
C
Y2
X2
Z2
D
Z2
Y2
X2
CorrectIncorrect -
Question 20 of 20
20. Question
20 Three different metals of the same size and shape, are added to dilute hydrochloric acid.
The diagram shows the results.
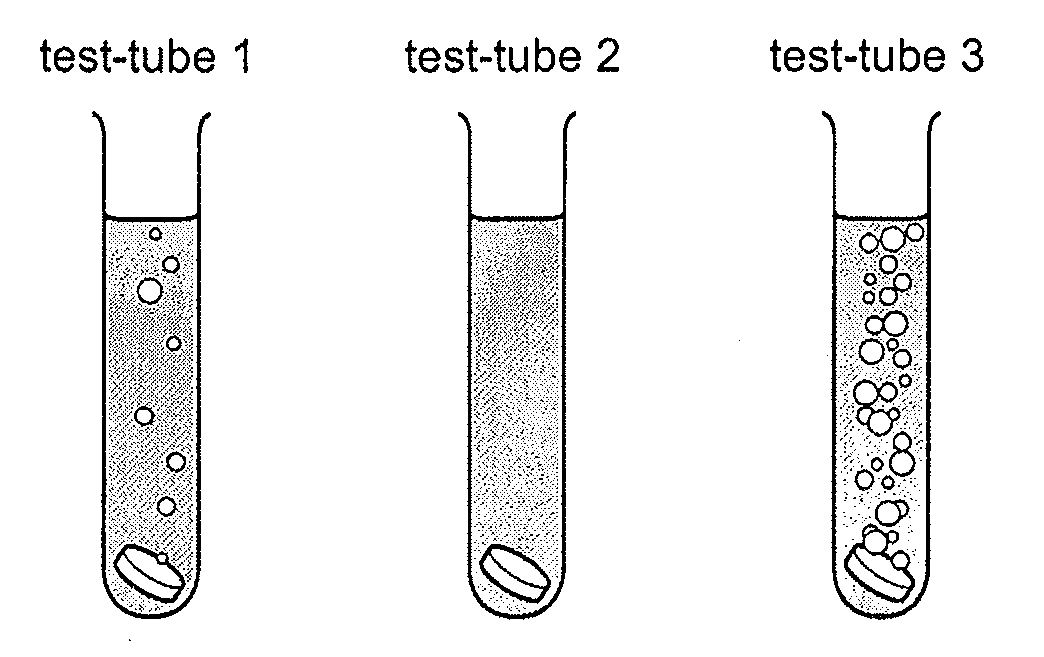
What could be the metals in the test tubes?
Test tube 1
Test tube 2
Test tube 3
A
copper
magnesium
iron
B
iron
copper
magnesium
C
magnesium
iron
copper
D
magnesium
copper
iron
CorrectIncorrect
