Quiz Summary
0 of 20 questions completed
Questions:
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- 11
- 12
- 13
- 14
- 15
- 16
- 17
- 18
- 19
- 20
Information
|
You must specify a text. |
|
|
You must specify a text. |
You have already completed the quiz before. Hence you can not start it again.
Quiz is loading…
You must sign in or sign up to start the quiz.
You must first complete the following:
Results
Results
0 of 20 questions answered correctly
Your time:
Time has elapsed
You have reached 0 of 0 point(s), (0)
Earned Point(s): 0 of 0, (0)
0 Essay(s) Pending (Possible Point(s): 0)
Categories
- Not categorized 0%
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- 11
- 12
- 13
- 14
- 15
- 16
- 17
- 18
- 19
- 20
- Answered
- Review
-
Question 1 of 20
1. Question
1 What is the volume of the liquid and which apparatus is used to measure it accurately?

apparatus
volume/cm3
A
burette
11.75
B
burette
10.25
C
measuring cylinder
10.20
D
measuring cylinder
10.25
CorrectIncorrect -
Question 2 of 20
2. Question
2 The melting and boiling points of four substances, R, S, T and U, are given in the table.
substance
melting point/ °C
boiling point/ °C
R
– 42
83
S
– 219
– 183
T
115 – 135
150
U
18
290
Which substances exist as liquids at 20 oC?
CorrectIncorrect -
Question 3 of 20
3. Question
3 The diagram shows an experiment.

What are the colours of the liquids in the flask and beaker at the end of the experiment?
flask
beaker
A
blue
blue
B
blue
colourless
C
colourless
blue
D
colourless
colourless
CorrectIncorrect -
Question 4 of 20
4. Question
In the following diagrams, each circle represents an atom. Different sized circles represent different atoms. Which diagram best represents a compound?
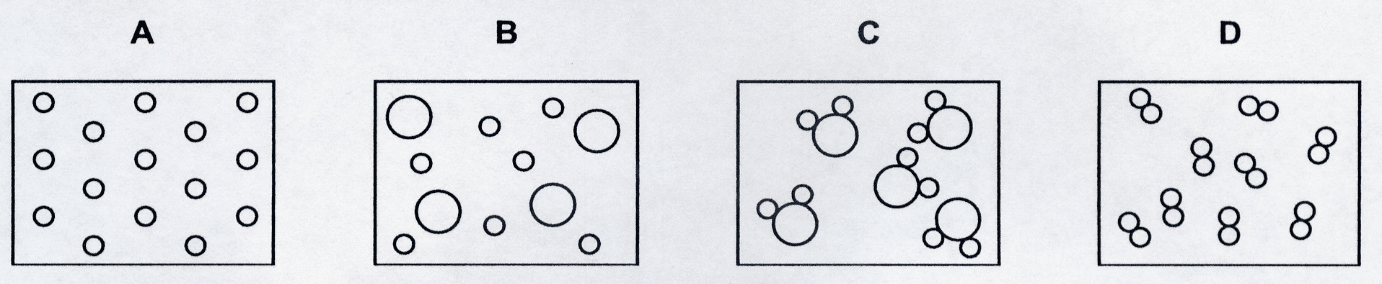 CorrectIncorrect
CorrectIncorrect -
Question 5 of 20
5. Question
Calcium fluoride has the formula CaF2. Which statement best describes the bond formed between the atoms?
CorrectIncorrect -
Question 6 of 20
6. Question
6 Two particles have the following composition, as shown in the table below.
particle
number of
electron
neutron
proton
X
12
12
12
Y
12
13
12
Particles X and Y
CorrectIncorrect -
Question 7 of 20
7. Question
Which substance is most likely to be a covalent molecule?
substance
boiling point / oC
solubility in water
electrical conductivity in liquid state
A
-85
soluble
none
B
-62
insoluble
none
C
1413
soluble
good
D
2977
insoluble
good
CorrectIncorrect -
Question 8 of 20
8. Question
Sodium metal reacts with water to produce a solution of sodium hydroxide and hydrogen. Which equation is balanced and shows the correct state symbols
A Na(s) + H2O(l) → NaOH(aq) + H2(g)
B Na(s) + H2O(aq) → NaOH(aq) + H2(g)
C 2Na(s) + 2H2O(aq) → 2NaOH(aq) + H2(g)
D 2Na(s) + 2H2O(l) → 2NaOH(aq) + H2(g)
CorrectIncorrect -
Question 9 of 20
9. Question
Which of the following oxides reacts with both hydrochloric acid and sodium hydroxide?
CorrectIncorrect -
Question 10 of 20
10. Question
Which two chemicals will react to make the salt, copper(II) sulfate?
CorrectIncorrect -
Question 11 of 20
11. Question
A drop of Universal Indicator is added to a solution of hydrochloric acid.
What colour change is observed?
CorrectIncorrect -
Question 12 of 20
12. Question
Which graph shows the trend in melting points of the Group I metals?
 CorrectIncorrect
CorrectIncorrect -
Question 13 of 20
13. Question
Code letters T, U, W, X, Y and Z of six different elements are placed in the Periodic Table shown below.
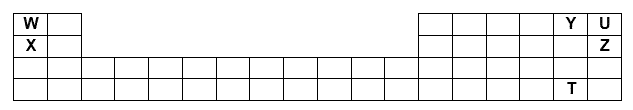
Which statement is true about the six elements?
CorrectIncorrect -
Question 14 of 20
14. Question
Garden tools are often galvanised to prevent steel from rusting. Galvanising involves coating the steel by dipping it in a molten metal. Which metal is used?
CorrectIncorrect -
Question 15 of 20
15. Question
The diagram shows the structure of brass.

Why is brass harder than pure copper?
CorrectIncorrect -
Question 16 of 20
16. Question
Over 90% of all gold but less than 50% of iron is recycled.
What is the reason for this difference?
CorrectIncorrect -
Question 17 of 20
17. Question
The reaction of water with four metals P, Q, R and S, is given in the table below.
metal
reaction with water
P
Reacts very readily with cold water.
Q
Reacts very slowly with cold water. Hot metal burns in steam with a brilliant white flame.
R
Explodes with cold water.
S
No reaction with hot or cold water.
Arrange P, Q, R and S in descending order of their reactivity.
CorrectIncorrect -
Question 18 of 20
18. Question
Magnesium phosphate has the chemical formula Mg3(PO4)2.
[Relative atomic masses: Ar: O, 16; Mg, 24; P, 31]
How should the relative formula mass, Mr, of magnesium phosphate be calculated?
CorrectIncorrect -
Question 19 of 20
19. Question
An element Z, forms an ion Z -. The electronic configuration of this ion is 2.8. Which statement is true about the element Z?
CorrectIncorrect -
Question 20 of 20
20. Question
Compound X is used to control soil acidity.
What is X?
CorrectIncorrect
