Quiz Summary
0 of 20 questions completed
Questions:
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- 11
- 12
- 13
- 14
- 15
- 16
- 17
- 18
- 19
- 20
Information
|
You must specify a text. |
|
|
You must specify a text. |
You have already completed the quiz before. Hence you can not start it again.
Quiz is loading…
You must sign in or sign up to start the quiz.
You must first complete the following:
Results
Results
0 of 20 questions answered correctly
Your time:
Time has elapsed
You have reached 0 of 0 point(s), (0)
Earned Point(s): 0 of 0, (0)
0 Essay(s) Pending (Possible Point(s): 0)
Categories
- Not categorized 0%
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- 11
- 12
- 13
- 14
- 15
- 16
- 17
- 18
- 19
- 20
- Answered
- Review
-
Question 1 of 20
1. Question
1 Which of the following pieces of apparatus is most suitable for accurately measuring out 16.80 cm3 of water?
 CorrectIncorrect
CorrectIncorrect -
Question 2 of 20
2. Question
2 A liquid that is used to flavour food is believed to be impure.
Which property would be the best way of testing its purity?A
boiling point
B
colour
C
density
D
solubility in water
CorrectIncorrect -
Question 3 of 20
3. Question
3 What happens to the molecules when steam condenses to water at room temperature?
A
Move faster and become closer together.
B
Move faster and become further apart.
C
Move slower and become closer together.
D
Move slower and become further apart.
CorrectIncorrect -
Question 4 of 20
4. Question
4 The following is a list of atomic number of some elements.
Which number belongs to the element that would not be found in the same group as the others?A
2
B
4
C
12
D
20
CorrectIncorrect -
Question 5 of 20
5. Question
The element oxygen can be represented as
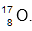 .
.
Which of the following shows the structure of the oxygen atom? CorrectIncorrect
CorrectIncorrect -
Question 6 of 20
6. Question
6 Oxygen forms a compound with sodium.
The element oxygen can be represented as
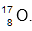 .
.Which of the following properties will the compound have?
A
It will be a good conductor of electricity in the solid state.
B
It will be a liquid at room temperature.
C
It will have a low melting point.
D
It will readily dissolve in water.
CorrectIncorrect -
Question 7 of 20
7. Question
7 An element X has a proton number 16 and nucleon number 34.
Which of the following statements is correct about element X?A
It conducts electricity in the liquid state.
B
It forms a basic oxide with oxygen.
C
It is an isotope of sulfur.
D
It is in Group IV and Period 3 of the Periodic Table.
CorrectIncorrect -
Question 8 of 20
8. Question
8 Which one of the following groups contains an element, a mixture and a compound?
A
chlorine, petroleum, salt water
B
copper, air, copper(II) sulfate
C
magnesium, lead, sulfur
D
pure water, carbon, magnesium
CorrectIncorrect -
Question 9 of 20
9. Question
9 Which pair of elements most likely form a covalent compound?
A
lithium and calcium
B
magnesium and sulfur
C
nitrogen and oxygen
D
sodium and nitrogen
CorrectIncorrect -
Question 10 of 20
10. Question
10 The relative formula mass, Mr, of ammonium nitrate, NH4NO3, is 80.
What mass of nitrogen is present in 160 g of ammonium nitrate?A
28
B
35
C
56
D
70
CorrectIncorrect -
Question 11 of 20
11. Question
11 Salt Y reacts with sodium hydroxide solution to produce a gas that turns damp red litmus paper blue.
Which of the following salts can salt Y be?A
ammonium sulfate
B
calcium sulfate
C
potassium sulfate
D
sodium sulfate
CorrectIncorrect -
Question 12 of 20
12. Question
12 Which of the following should not be used with dilute sulfuric acid to prepare iron(II) sulfate?
A
iron
B
iron(II) carbonate
C
iron(II) nitrate
D
iron(II) oxide
CorrectIncorrect -
Question 13 of 20
13. Question
13 Which of the following oxides does not react with dilute hydrochloric acid to form a salt and water?
A
aluminium oxide
B
lead(II) oxide
C
magnesium oxide
D
nitrogen dioxide
CorrectIncorrect -
Question 14 of 20
14. Question
14 Chlorine gas was bubbled into aqueous potassium iodide as shown in the equation
below:Cl2 (g) + 2KI (aq) → 2KCl (aq) + I2 (aq)
Which of the following statements are correct for this reaction?
I Chlorine is more reactive than iodine.
II Iodine is displaced.
III The colourless solution turns brown.
A
I and II only
B
I and III only
C
II and III only
D
I, II and III
CorrectIncorrect -
Question 15 of 20
15. Question
15 Which substance helps to remove acidic impurities during the extraction of iron in the blast furnace?
A
carbon
B
limestone
C
nitrogen
D
slag
CorrectIncorrect -
Question 16 of 20
16. Question
16 Which of the following describes the most common method used for rust prevention in each case?
food containers
metal bridges
steel machinery
A
coated with tin
coated with paint
coated with oil
B
coated with oil
coated with tin
coated with paint
C
coated with oil
coated with paint
coated with tin
D
coated with paint
coated with oil
coated with tin
CorrectIncorrect -
Question 17 of 20
17. Question
17 Element X reacts very slowly with cold water, but reacts vigorously with steam.
Which of the following statements is true?A
It is less reactive than copper.
B
It is less reactive than iron.
C
It is more reactive than calcium.
D
It is more reactive than silver.
CorrectIncorrect -
Question 18 of 20
18. Question
18 Which air pollutant is not correctly matched to its source?
air pollutant
source
A
methane
decay of rubbish in landfill
B
nitrogen oxides
lightning activity
C
sulfur dioxide
volcanoes
D
unburned hydrocarbons
complete combustion of fossil fuels
CorrectIncorrect -
Question 19 of 20
19. Question
19 In the fractional distillation of crude oil, which of the following fractions has the lowest boiling point?
A
bitumen
B
kerosene
C
diesel
D
lubricating oil
CorrectIncorrect -
Question 20 of 20
20. Question
20 Which property of alkanes does not increase as relative molecular mass increases?
A
boiling point
B
flammability
C
melting point
D
viscosity
CorrectIncorrect
